argenx SE (Euronext & Nasdaq: ARGX), a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases, today announced its half year 2025 results and provided a second quarter business update.
“We continue to make meaningful progress towards our Vision 2030, advancing bold innovation that has already reached more than 15,000 patients globally” said Tim Van Hauwermeiren, Chief Executive Officer of argenx. “VYVGART is delivering strong growth across all indications, formulations and regions. We are still in the early stages of capturing the full market opportunity in MG and CIDP, with the recent launch of the VYVGART SC prefilled syringe driving demand from new patients and prescribers. In MG, we are shaping the market as the fastest growing biologic, moving earlier in the patient treatment paradigm, and working toward the broadest possible label. In CIDP, we continue to see consistent patient growth, with ample runway to reach the 12,000 patients in the U.S. who remain inadequately controlled on standard of care. This is just the beginning of the larger growth opportunity ahead. With six registrational and six proof-of-concept readouts expected by the end of 2026, we are executing on our proven innovation playbook that is delivering pipeline-in-a-product opportunities aimed at transforming care for patients with high unmet need.”
Advancing Towards Vision 2030
argenx has established its strategic priorities to advance Vision 2030, aiming to treat 50,000 patients globally with its medicines, secure 10 labeled indications across all approved medicines, and advance five pipeline candidates into Phase 3 development by 2030.
Expand global VYVGART opportunity and launch VYVGART SC as prefilled syringe
VYVGART® (IV: efgartigimod alfa-fcab and SC: efgartigimod alfa and hyaluronidase-qvfc) is a first-and-only IgG Fc-antibody fragment that targets the neonatal Fc receptor (FcRn). It is approved in three indications, including generalized myasthenia gravis (gMG) globally, primary immune thrombocytopenia (ITP) in Japan, and chronic inflammatory demyelinating polyneuropathy (CIDP) in the U.S., Japan, China, and the EU. The VYVGART-SC prefilled syringe (PFS) is now approved for use in the U.S. and EU.
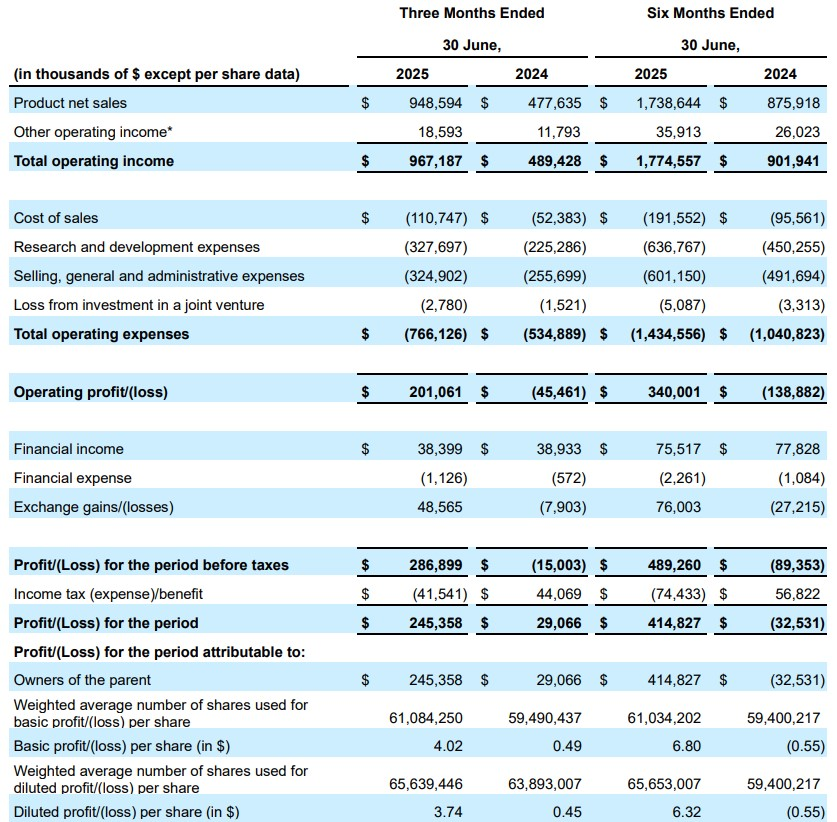
• Generated global product net sales (inclusive of both VYVGART and VYVGART SC) of $949 million in the second quarter of 2025
◦ Strong underlying fundamentals across key patient and prescriber metrics with 97% operational growth in product net sales year-over-year from second quarter 2024, and 19% from the first quarter of 2025
• First patient dosed in Germany following European Commission (EC) approval for VYVGART-SC (vial and PFS) for CIDP
• PFS decision on approval for gMG and CIDP expected in Japan and Canada by end of year
• Evidence generation through label-enabling studies:
◦ Topline results expected in second half of 2025 for seronegative gMG (ADAPT-SERON) and first half of 2026 for ocular MG (ADAPT OCULUS)
◦ Topline results expected in second half of 2026 to support FDA submission of VYVGART IV for primary ITP (ADVANCE-NEXT)
Execute 10 registrational and 10 proof-of-concept studies across efgartigimod, empasiprubart and ARGX-119 to advance the next wave of launches
argenx continues to demonstrate breadth and depth within its immunology pipeline, advancing multiple first-in-class product candidates with potential across high-need indications.
Efgartigimod Development
Efgartigimod is being studied across 15 severe autoimmune diseases, highlighting the broad potential of FcRn biology in neurology, rheumatology, and beyond.
• Registrational studies are currently ongoing in idiopathic inflammatory myopathies (IIM or myositis), thyroid eye disease (TED), and Sjögren’s disease
◦ Topline results from ALKIVIA study evaluating three myositis subsets (immune-mediated necrotizing myopathy (IMNM), anti-synthetase syndrome (ASyS) and dermatomyositis (DM)) expected in second half of 2026
◦ Topline results from two registrational UplighTED studies (TED) expected in second half of 2026
◦ Topline results from registrational UNITY study (Sjögren’s disease) expected in 2027
• Proof-of-concept studies ongoing in lupus nephritis (LN), systemic sclerosis (SSc) and antibody mediated rejection (AMR); topline results expected for LN in fourth quarter of 2025, SSc in second half of 2026, and AMR in 2027
Empasiprubart Development
Empasiprubart, a first-in-class, humanized, monoclonal antibody that specifically binds to C2, is currently being evaluated in four indications. These include registrational studies in multifocal motor neuropathy (MMN) and CIDP, and proof-of-concept studies in delayed graft function (DGF) and DM.
• Topline results from registrational EMPASSION study (MMN) evaluating empasiprubart head-to-head versus IVIg expected in second half of 2026
• Registrational EMVIGORATE study ongoing in CIDP evaluating empasiprubart head-to-head versus IVIg
• Topline results expected for DGF in the second half of 2025 and for DM in first half of 2026
ARGX-119 Development
ARGX-119, a first-in-class agonist antibody that targets muscle-specific kinase (MuSK), is being evaluated in congenital myasthenic syndromes (CMS), amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA).
• Registrational study to start in CMS in 2026 following positive Phase 1b proof-of-concept data
• Phase 2a proof-of-concept study ongoing in ALS; topline results expected in first half of 2026
• SMA proof-of-concept study on track to start by end of year
• ARGX-119 R&D webinar to be hosted on September 16, 2025
Advance four new pipeline molecules and generate sustainable value through continued investment in Immunology Innovation Program
argenx continues to invest in its Immunology Innovation Program (IIP) to drive long-term sustainable pipeline growth. Through the IIP, four new pipeline candidates have been nominated, including: ARGX-213, targeting FcRn and further solidifying argenx’s leadership in this biology; ARGX-121, a first-in-class molecule targeting IgA; ARGX-109, targeting IL-6, which plays an important role in inflammation, and a fourth pipeline candidate, a first-in-class sweeping antibody for which the target has not yet been disclosed.
• Phase 1 results from ongoing ARGX-109 study expected in second half of 2025, and from ongoing ARGX-213 and ARGX-121 studies expected in first half of 2026
• Entered strategic collaboration with Unnatural Products (UNP) to expand argenx discovery capabilities into the oral peptide space. This partnership reinforces argenx's commitment to enhance the patient experience and advance its pipeline of precision therapies.