argenx SE (Euronext & Nasdaq: ARGX), a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases, today announced its third quarter 2024 financial results and provided a business update.
“We delivered significant patient impact with VYVGART over the quarter, expanding our gMG footprint and delivering innovation to CIDP patients three months into launch,” said Tim Van Hauwermeiren, Chief Executive Officer of argenx. “We continued to advance our goal of reaching more gMG patients earlier in their treatment journey, supported by VYVGART’s strong safety and efficacy profile, and real-world data showing the ability to meaningfully reduce steroid use. Expanding upon our leadership in gMG, we are now paving the future in CIDP. The strength of our data, combined with execution across the team to reach key stakeholders, contributed to the initial success of our CIDP launch, with more than 300 patients on therapy at the end of the third quarter. There remains significant opportunity ahead as we work towards achieving our Vision 2030, with innovation implemented across our pipeline to deliver transformative outcomes to more patients.”
Advancing ‘Vision 2030’ in Third Quarter 2024
Vision 2030 is the next phase in argenx’s long-term commitment to transform the treatment of autoimmune diseases by strengthening its leadership in FcRn biology, investing in its continuous pipeline of differentiated antibody candidates, and scaling in a disciplined way to ensure innovation remains core to the argenx mission. As a part of this vision, argenx plans to reach at least 50,000 patients globally, advance the pipeline to achieve 10 labeled indications, and bring five new molecules into Phase 3 by 2030.
Reaching 50,000 Patients Globally by 2030
VYVGART® (efgartigimod alfa-fcab) is a first-in-class antibody fragment targeting FcRn and is now approved for both intravenous use and subcutaneous injection (SC) (efgartigimod alfa and hyaluronidase-qvfc) in three indications, including generalized myasthenia gravis (gMG) globally, primary immune thrombocytopenia (ITP) in Japan (IV only), and chronic inflammatory demyelinating polyneuropathy (CIDP) in the U.S. (SC only).
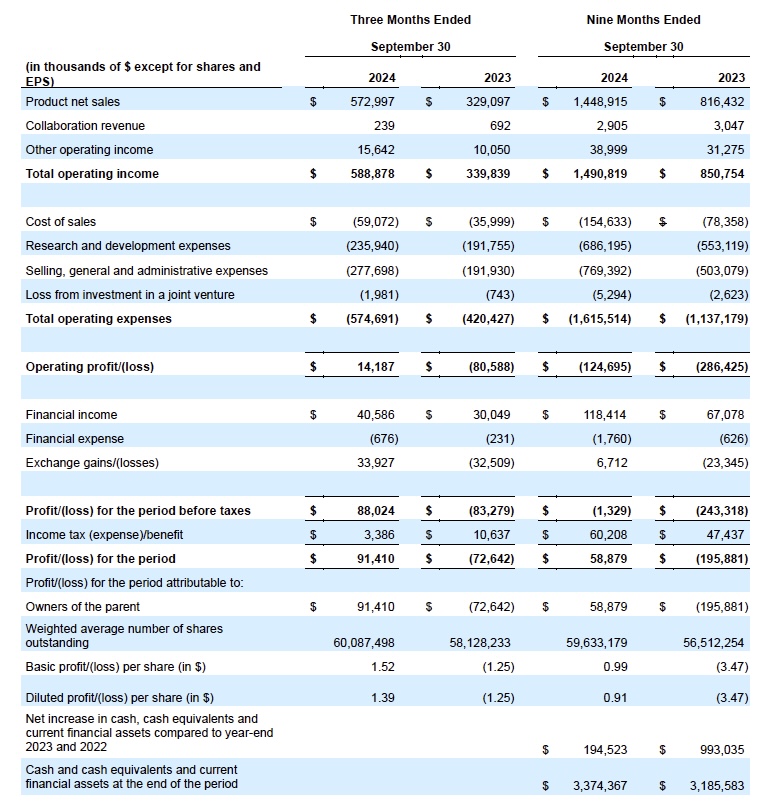
- Generated global net product revenues (inclusive of both VYVGART and VYVGART SC) of $573 million in the third quarter of 2024
- Multiple VYVGART regulatory submissions completed or underway for gMG, including:
- Swissmedic approved VYVGART for the treatment of gMG in Switzerland
- Regulatory decisions on approval expected in Australia and Saudi Arabia in 2024, and South Korea in 2025
- Multiple VYVGART SC regulatory submissions under review or planned for CIDP, including:
- Regulatory submissions completed in Japan, Europe, and China with decisions on approval expected in 2025
- Regulatory submission to be completed in Canada by end of 2024
- VYVGART now reimbursed in 11 countries in Europe, with new agreements in place in France, Luxembourg, and Belgium
- FDA review of VYVGART SC pre-filled syringe (PFS) for gMG and CIDP ongoing with Prescription Drug User Fee Act (PDUFA) target action date of April 10, 2025
Advancing Pipeline to Achieve 10 Labeled Indications by 2030
argenx continues to demonstrate breadth and depth within its immunology pipeline, advancing multiple pipeline- in-a-product candidates. argenx is solidifying its leadership in FcRn biology, with efgartigimod currently in development in 15 indications. argenx is also advancing its first-in-class C2 inhibitor, empasiprubart, which is being evaluated in multifocal motor neuropathy (MMN), delayed graft function (DGF), dermatomyositis (DM), and CIDP. In addition, argenx is evaluating ARGX-119, a muscle-specific kinase (MuSK) agonist in both congenital myasthenic syndrome (CMS) and amyotrophic lateral sclerosis (ALS).
- Registrational studies ongoing of efgartigimod in thyroid eye disease (TED)
- Registrational studies ongoing to support label-expansion into broader MG, including ADAPT SERON in seronegative gMG and ADAPT OCULUS in ocular MG
- Registrational study in primary Sjögren’s disease (SjD) on track to start by end of 2024
- Confirmatory study of efgartigimod in primary ITP to start by end of 2024 to enable registration in U.S.
- Topline data from seamless Phase 2/3 ALKIVIA study evaluating efgartigimod across three myositis subsets (immune-mediated necrotizing myopathy (IMNM), anti-synthetase syndrome (ASyS), and DM) expected by end of 2024
- Update on BALLAD study development plan evaluating efgartigimod in bullous pemphigoid (BP) expected by end of 2024
- Decision made to discontinue development of efgartigimod in membranous nephropathy (MN); proof-of concept study ongoing with efgartigimod in lupus nephritis (LN)
- Proof-of-concept study ongoing with efgartigimod in antibody mediated rejection (AMR), with systemic sclerosis (SSc) to start by end of 2024
- Registrational study of empasiprubart in MMN to start by end of 2024
- Additional proof-of-concept studies of empasiprubart ongoing, including VARVARA study in DGF and EMPACIFIC study in DM
- Registrational study of empasiprubart in CIDP to start in 2025
- Ongoing Phase 1b/2a studies of ARGX-119 to assess early signal in patients with CMS and ALS
Investing in Immunology Innovation Program to Support Five New Molecules in Phase 3 by 2030
argenx continues to invest in its Immunology Innovation Program (IIP) to drive long-term sustainable pipeline growth. Through the IIP, four new pipeline candidates have been nominated, including: ARGX-213, targeting FcRn and further solidifying argenx’s leadership in this new class of medicine; ARGX-121, a first-in-class molecule targeting IgA; ARGX-109, targeting IL-6, which plays an important role in inflammation, and ARGX-220, a first-in- class sweeping antibody for which the target has not yet been disclosed.
- Phase 1 studies of ARGX-213 and ARGX-121 expected to start in second half of 2025
- Investigational new drug (IND) applications for ARGX-220 and ARGX-109 on track to be filed by end of 2025
THIRD QUARTER 2024 FINANCIAL RESULTS
argenx SE
UNAUDITED CONDENSED CONSOLIDATED INTERIM STATEMENTS OF PROFIT OR LOSS